Sitemap
A list of all the posts and pages found on the site. For you robots out there is an XML version available for digesting as well.
Pages
Posts
portfolio
Portfolio item number 1
Short description of portfolio item number 1
Portfolio item number 2
Short description of portfolio item number 2 
publications
Engineering a Functional Small RNA Negative Autoregulation Network with Model-Guided Design
Published in ACS Synthetic Biology, 2018
RNA regulators are powerful components of the synthetic biology toolbox. Here, we expand the repertoire of synthetic gene networks built from these regulators by constructing a transcriptional negative autoregulation (NAR) network out of small RNAs (sRNAs). NAR network motifs are core motifs of natural genetic networks, and are known for reducing network response time and steady state signal. Here we use cell-free transcription–translation (TX-TL) reactions and a computational model to design and prototype sRNA NAR constructs. Using parameter sensitivity analysis, we design a simple set of experiments that allow us to accurately predict NAR function in TX-TL. We transfer successful network designs into Escherichia coli and show that our sRNA transcriptional network reduces both network response time and steady-state gene expression. This work broadens our ability to construct increasingly sophisticated RNA genetic networks with predictable function.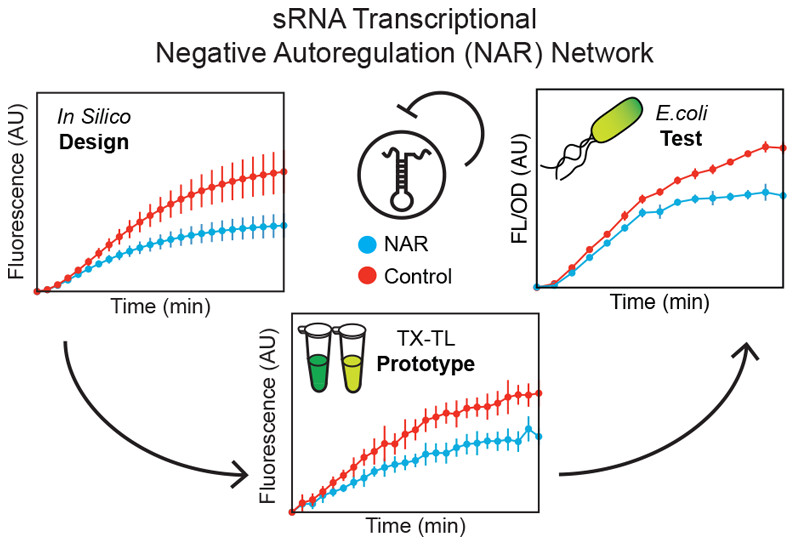
Recommended citation: Chelsea Hu, Melissa Takahashi, Yan Zhang, Julius Lucks, "Engineering a Functional Small RNA Negative Autoregulation Network with Model-Guided Design." ACS Synthetic Biology, 2018.
Download Paper
Point-of-care biomarker quantification enabled by sample-specific calibration
Published in Science Advances, 2019
Easy-to-perform, relatively inexpensive blood diagnostics have transformed at-home healthcare for some patients, but they require analytical equipment and are not easily adapted to measuring other biomarkers. The requirement for reliable quantification in complex sample types (such as blood) has been a critical roadblock in developing and deploying inexpensive, minimal-equipment diagnostics. Here, we developed a platform for inexpensive, easy-to-use diagnostics that uses cell-free expression to generate colored readouts that are visible to the naked eye, yet quantitative and robust to the interference effects seen in complex samples. We achieved this via a parallelized calibration scheme that uses the patient sample to generate custom reference curves. We used this approach to quantify a clinically relevant micronutrient and to quantify nucleic acids, demonstrating a generalizable platform for low-cost quantitative diagnostics.
Recommended citation: Monica McNerney, Yan Zhang, Paige Steppe, Adam Silverman, Michael Jewett, Mark Styczynski, "Point-of-care biomarker quantification enabled by sample-specific calibration." Science Advances, 2019.
Download Paper
ElectroPen: An ultra-low–cost, electricity-free, portable electroporator
Published in PLOS Biology, 2020
Electroporation is a basic yet powerful method for delivering small molecules (RNA, DNA, drugs) across cell membranes by application of an electrical field. It is used for many diverse applications, from genetically engineering cells to drug- and DNA-based vaccine delivery. Despite this broad utility, the high cost of electroporators can keep this approach out of reach for many budget-conscious laboratories. To address this need, we develop a simple, inexpensive, and handheld electroporator inspired by and derived from a common household piezoelectric stove lighter. The proposed "ElectroPen" device can cost as little as 23 cents (US dollars) to manufacture, is portable (weighs 13 g and requires no electricity), can be easily fabricated using 3D printing, and delivers repeatable exponentially decaying pulses of about 2,000 V in 5 ms. We provide a proof-of-concept demonstration by genetically transforming plasmids into Escherichia coli cells, showing transformation efficiency comparable to commercial devices, but at a fraction of the cost. We also demonstrate the potential for rapid dissemination of this approach, with multiple research groups across the globe validating the ease of construction and functionality of our device, supporting the potential for democratization of science through frugal tools. Thus, the simplicity, accessibility, and affordability of our device holds potential for making modern synthetic biology accessible in high school, community, and resource-poor laboratories.
Recommended citation: Gaurav Byagathvalli, Soham Sinha, Yan Zhang, Mark Styczynski, Janet Standeven, M. Bhamla, "ElectroPen: An ultra-low–cost, electricity-free, portable electroporator." PLOS Biology, 2020.
Download Paper
Metabolic Dynamics in Escherichia coli-Based Cell-Free Systems
Published in ACS Synthetic Biology, 2021
The field of metabolic engineering has yielded remarkable accomplishments in using cells to produce valuable molecules, and cell-free expression (CFE) systems have the potential to push the field even further. However, CFE systems still face some outstanding challenges, including endogenous metabolic activity that is poorly understood yet has a significant impact on CFE productivity. Here, we use metabolomics to characterize the temporal metabolic changes in CFE systems and their constituent components, including significant metabolic activity in central carbon and amino acid metabolism. We find that while changing the reaction starting state via lysate preincubation impacts protein production, it has a comparatively small impact on metabolic state. We also demonstrate that changes to lysate preparation have a larger effect on protein yield and temporal metabolic profiles, though general metabolic trends are conserved. Finally, while we improve protein production through targeted supplementation of metabolic enzymes, we show that the endogenous metabolic activity is fairly resilient to these enzymatic perturbations. Overall, this work highlights the robust nature of CFE reaction metabolism as well as the importance of understanding the complex interdependence of metabolites and proteins in CFE systems to guide optimization efforts.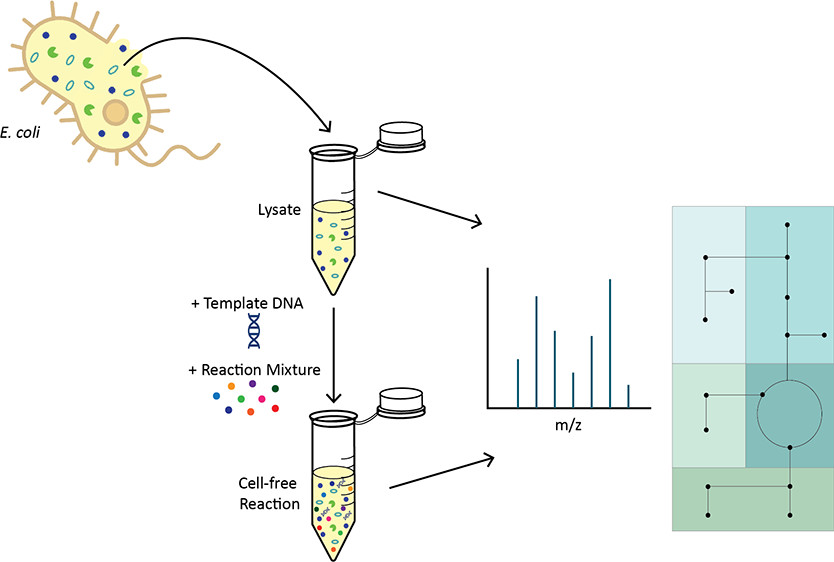
Recommended citation: April Miguez, Yan Zhang, Fernanda Piorino, Mark Styczynski, "Metabolic Dynamics in Escherichia coli-Based Cell-Free Systems." ACS Synthetic Biology, 2021.
Download Paper
Protocell arrays for simultaneous detection of diverse analytes
Published in Nature Communications, 2021
Simultaneous detection of multiple analytes from a single sample (multiplexing), particularly when done at the point of need, can guide complex decision-making without increasing the required sample volume or cost per test. Despite recent advances, multiplexed analyte sensing still typically faces the critical limitation of measuring only one type of molecule (e.g., small molecules or nucleic acids) per assay platform. Here, we address this bottleneck with a customizable platform that integrates cell-free expression (CFE) with a polymer-based aqueous two-phase system (ATPS), producing membrane-less protocells containing transcription and translation machinery used for detection. We show that multiple protocells, each performing a distinct sensing reaction, can be arrayed in the same microwell to detect chemically diverse targets from the same sample. Furthermore, these protocell arrays are compatible with human biofluids, maintain function after lyophilization and rehydration, and can produce visually interpretable readouts, illustrating this platform’s potential as a minimal-equipment, field-deployable, multi-analyte detection tool.
Recommended citation: Yan Zhang, Taisuke Kojima, Ge-Ah Kim, Monica McNerney, Shuichi Takayama, Mark Styczynski, "Protocell arrays for simultaneous detection of diverse analytes." Nature Communications, 2021.
Download Paper
Point-of-Care Analyte Quantification and Digital Readout via Lysate-Based Cell-Free Biosensors Interfaced with Personal Glucose Monitors
Published in ACS Synthetic Biology, 2021
Field-deployable diagnostics based on cell-free systems have advanced greatly, but on-site quantification of target analytes remains a challenge. Here we demonstrate that Escherichia coli lysate-based cell-free biosensors coupled to a personal glucose monitor (PGM) can enable on-site analyte quantification, with the potential for straightforward reconfigurability to diverse types of analytes. We show that analyte-responsive regulators of transcription and translation can modulate the production of the reporter enzyme β-galactosidase, which in turn converts lactose into glucose for PGM quantification. Because glycolysis is active in the lysate and would readily deplete converted glucose, we decoupled enzyme production and glucose conversion to increase the end point signal output. However, this lysate metabolism did allow for one-pot removal of glucose present in complex samples (like human serum) without confounding target quantification. Taken together, our results show that integrating lysate-based cell-free biosensors with PGMs enables accessible target detection and quantification at the point of need.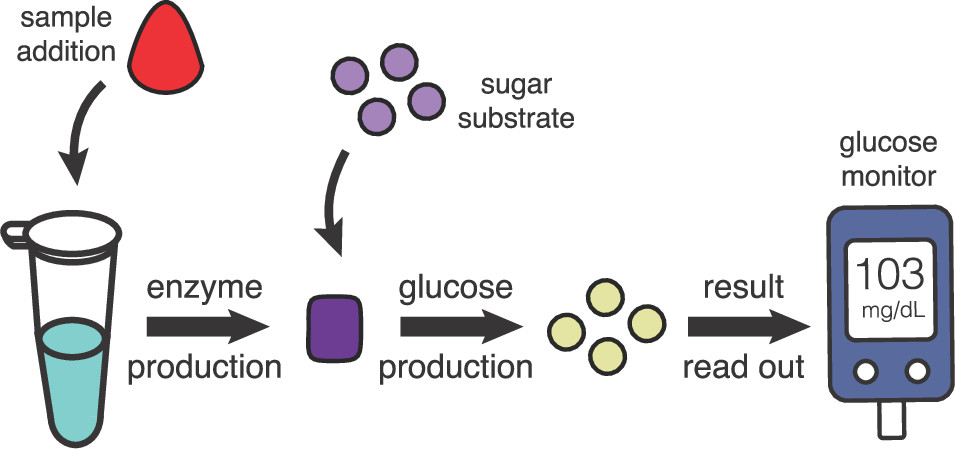
Recommended citation: Yan Zhang, Paige Steppe, Maxwell Kazman, Mark Styczynski, "Point-of-Care Analyte Quantification and Digital Readout via Lysate-Based Cell-Free Biosensors Interfaced with Personal Glucose Monitors." ACS Synthetic Biology, 2021.
Download Paper
Nucleic Acid Partitioning in PEG-Ficoll Protocells
Published in Journal of Chemical & Engineering Data, 2022
The phase separation of aqueous polymer solutions is a widely used method for producing self-assembled, membraneless droplet protocells. Nonionic synthetic polymers forming an aqueous two-phase system (ATPS) have been shown to reliably form protocells that, when equipped with biological materials, are useful for applications such as analyte detection. Previous characterization of an ATPS-templated protocell did not investigate the effects of its biological components on phase stability. Here we report the phase diagram of a PEG 35k-Ficoll 400k-water ATPS at baseline and in the presence of necessary protocell components. Because the stability of an ATPS can be sensitive to small changes in composition, which in turn impacts solute partitioning, we present partitioning data of a variety of nucleic acids in response to protocell additives. The results show that the additives─particularly a mixture of salts and small organic molecules─have profound positive effects on ATPS stability and nucleic acid partitioning, both of which significantly contribute to protocell function. Our data uncovers several new areas of optimization for future protocell engineering.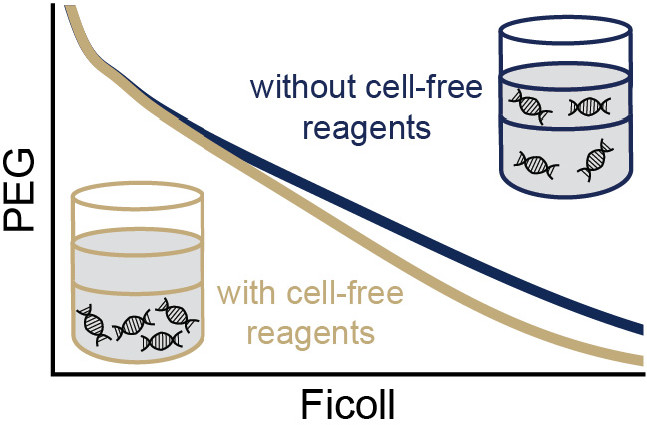
Recommended citation: Tasdiq Ahmed, Yan Zhang, Ji-Hoon Lee, Mark Styczynski, Shuichi Takayama, "Nucleic Acid Partitioning in PEG-Ficoll Protocells." Journal of Chemical & Engineering Data, 2022.
Download Paper
Short Activators and Repressors of RNA Toehold Switches
Published in ACS Synthetic Biology, 2023
RNA toehold switches are a widely used class of molecule to detect specific RNA trigger sequences, but their design, intended function, and characterization to date leave it unclear whether they can function properly with triggers shorter than 36 nucleotides. Here, we explore the feasibility of using standard toehold switches with 23-nucleotide truncated triggers. We assess the crosstalk of different triggers with significant homology and identify a highly sensitive trigger region where just one mutation from the consensus trigger sequence can reduce switch activation by 98.6%. However, we also find that triggers with as many as seven mutations outside of this region can still lead to 5-fold induction of the switch. We also present a new approach using 18- to 22-nucleotide triggers as translational repressors for toehold switches and assess the off-target regulation for this strategy as well. The development and characterization of these strategies could help enable applications like microRNA sensors, where well-characterized crosstalk between sensors and detection of short target sequences are critical. 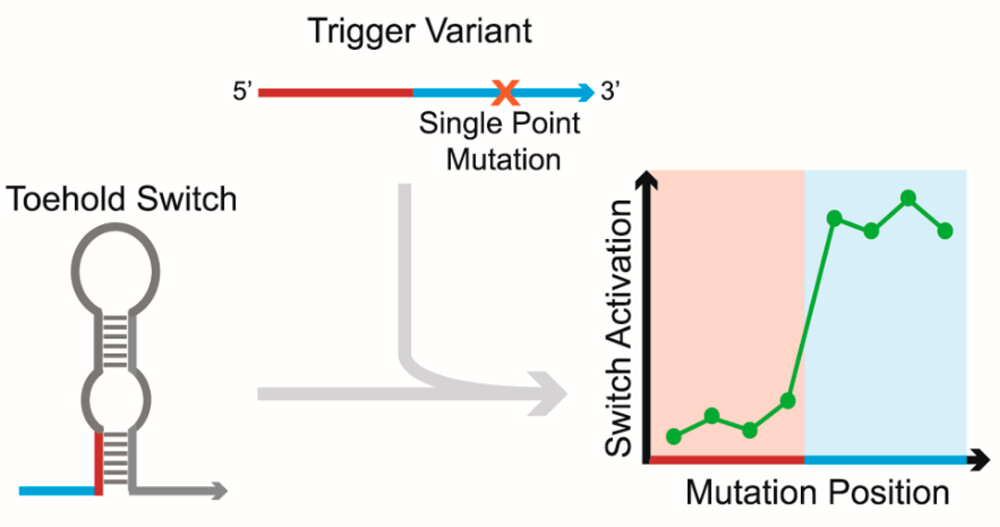
Recommended citation: Megan McSweeney, Yan Zhang, Mark Styczynski, "Short Activators and Repressors of RNA Toehold Switches." ACS Synthetic Biology, 2023.
Download Paper
Optimizing protein production in the One-Pot Pure system: insights into reaction composition and expression efficiency
Published in ACS Synthetic Biology, 2025
The One-Pot PURE (Protein synthesis Using Recombinant Elements) system simplifies the preparation of traditional PURE systems by coculturing and purifying 36 essential proteins for gene expression in a single step, enhancing accessibility and affordability for widespread laboratory adoption and customization. However, replicating this protocol to match the productivity of traditional PURE systems can take considerable time and effort due to uncharacterized variability. In this work, we observed unstable PURE protein expression in the original One-Pot PURE strains, E. coli M15/pREP4 and BL21(DE3), and addressed this issue using glucose-mediated catabolite repression to minimize burdensome background expression. We also identified several limitations making the M15/pREP4 strain unsuitable for PURE protein expression, including coculture incompatibility with BL21(DE3) and uncharacterized proteolytic activity. We showed that consolidating all expression vectors into a protease-deficient BL21(DE3) strain minimized proteolysis, led to more uniform coculture cell growth at the time of induction, and improved the stoichiometry of critical translation initiation factors in the final PURE mixture for efficient cell-free protein production. In addition to optimizing the One-Pot PURE protein composition, we found that variations in commercial energy solution formulations could compensate for suboptimal PURE protein stoichiometry. Notably, altering the source of E. coli tRNAs in the energy solution alone led to significant differences in the expression capacity of cell-free reactions, highlighting the importance of tRNA codon usage in influencing protein expression yield. Taken together, this work systematically investigates the proteome and biochemical factors influencing the One-Pot PURE system productivity, offering insights to enhance its robustness and adaptability across laboratories. 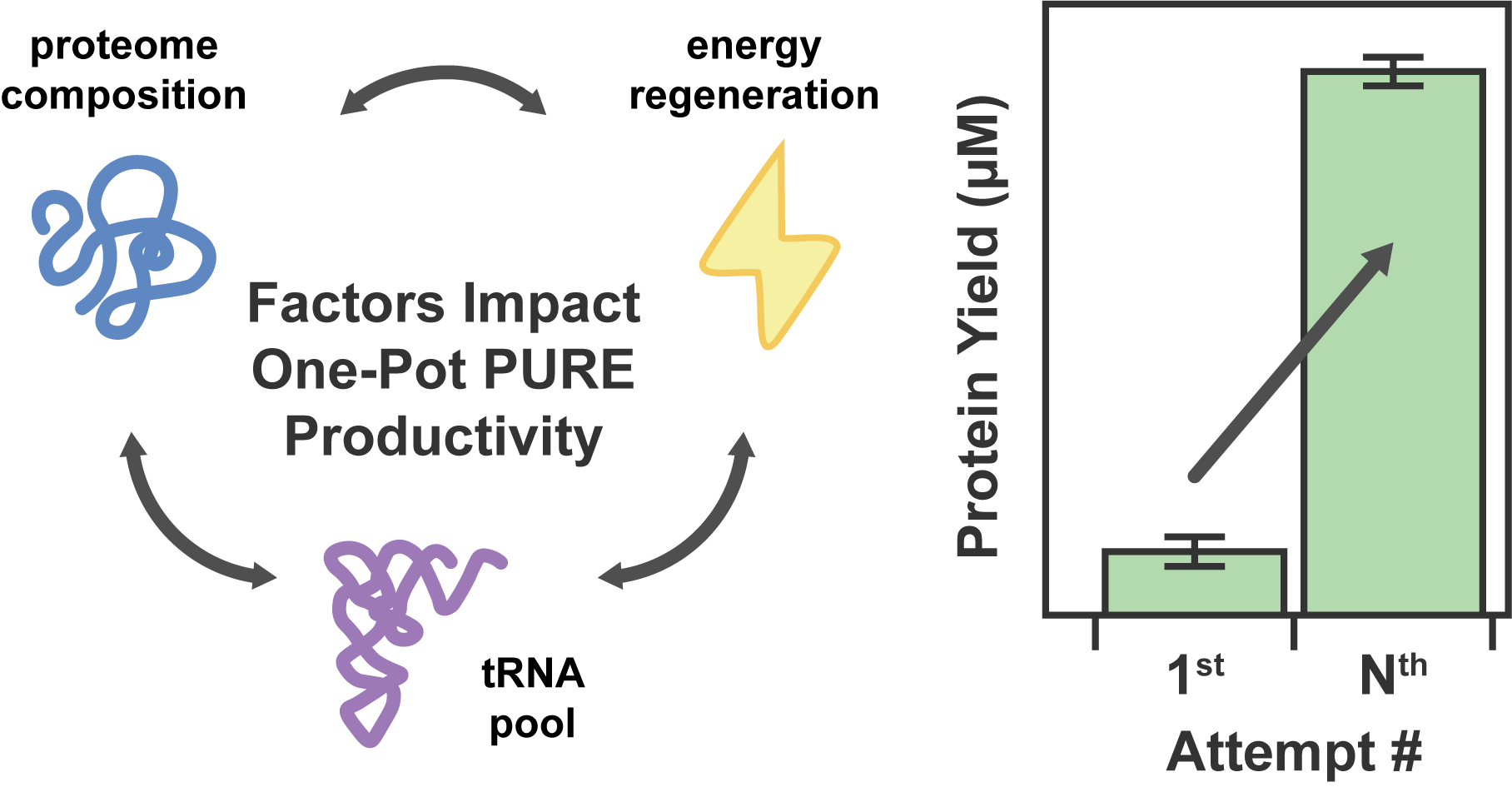
Recommended citation: Yan Zhang, Matas Deveikis, Yanping Qiu, Lovisa Björn, Zachary A. Martinez, Tsui-Fen Chou, Paul S. Freemont, Richard M. Murray, " Optimizing protein production in the One-Pot Pure system: insights into reaction composition and expression efficiency. " ACS Synthetic Biology, 2025.
Download Paper
talks
teaching
Teaching experience 1
Undergraduate course, University 1, Department, 2014
This is a description of a teaching experience. You can use markdown like any other post.
Teaching experience 2
Workshop, University 1, Department, 2015
This is a description of a teaching experience. You can use markdown like any other post.

